
The research has achieved great breakthrough and got the support from NSFC ( Natural Science Foundation of China ).

Recently, new energy materials research institute of Tianjin university and the key laboratory of boron nitride materials of Hebei university of technology, work in close cooperation and make important progress in terms of cubic boron nitride synthesis. The research papers "photochemical short of ultrafine cubic boron nitride nanoparticles at ambient the conditions", has been published in chemical top journal Applied Chemistry of Germany, and was elected as a hot thesis.
Cubic boron nitride is a kind of high hardness material, whose hardness will rise sharply with the decrease of the size, and it is widely used in cutting tool materials, wear resistant materials and high temperature areas. But cubic boron nitride will remain stable only at high pressure. Usually, synthesis of cubic boron nitride need high pressure (hundreds of thousands of atmospheric pressure), high temperature (1000 to 2000 degrees Celsius) and a long time (a few hours to several days), and so far, the minimum synthetic cubic boron nitride particle size is 14 nm.
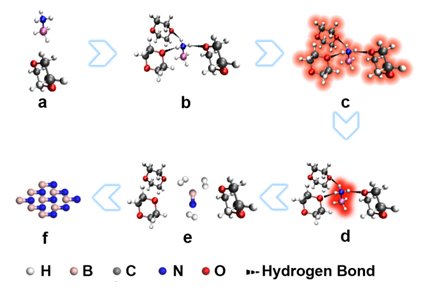
The research team of Du Xiwen in Tianjin university obtains many achievements of pioneering in the field of laser composite materials, and takes the lead in realizing the long pulse width laser controlled synthesis of nanomaterials. And Tang Chengchun team of Hebei University of Technology also has achieved green synthesis, performance research and application development of the new materials of boron nitride. Based on their respective advantages, they both start to study the laser synthesis of cubic boron nitride, try a variety of process routes and material systems, and finally get the synthesis of ultrafine (3.5 nm) cubic boron nitride nanoparticles by using laser irradiation, ammonia borane solution under atmospheric pressure. Studies have been found that an ammonia borane with three polar solvent molecules can combine to form a primitive, which can also absorb the four laser photons, transfer energy to the ammonia borane molecules, undermine its chemical bonds, and achieve the complete dehydrogenation. Under the action of a strong laser, large amounts of boron nitride molecules will be produced in solution, which will cause the explosive nucleation of boron nitride particles and then obtain ultrafine boron nitride particles. Large radius of curvature of ultrafine nanoparticles can produce additional surface pressure, which can make the cubic phase stable at room temperature. Finally it will get the cubic boron nitride particles.
The research has achieved great breakthrough as follows: 1) It firstly reported the synthesis of cubic boron nitride at room temperature under atmospheric pressure, and the speed of synthesis is so fast , and the entire process only takes 10 minutes. 2) It is for the first time that the synthesis of ultrafine cubic boron nitride particles is compounded, whose size is only 3.5 nm and hardness should reach the peak. 3) It implements the complete dehydrogenation of ammonia borane and provides a new technology to desorb hydrogen quickly for hydrogen storage material.
The study has got the support from NSFC ( Natural Science Foundation of China ).