
A new study from MIT could keep lithium ion battery technology on the track for another few laps, allowing further improvements while we wait for a fundamentally better solution to arrive.

A new study from MIT could keep lithium ion battery technology on the track for another few laps, allowing further improvements while we wait for a fundamentally better solution to arrive. The breakthrough comes from an accidentally created synthetic metal nanoparticle that could solve some of the oldest problems for batteries. Their testing shows that the nanoparticles could allow up to four times the charge retention after a long lifetime of use, meaning devices could last longer and create far less unnecessary pollution.
Not long ago, scientists discovered that the main reason lithium ion batteries lose their capacity over many charge-discharge cycles has to do with expansion and contraction of the graphite electrodes at either end. When electron-laden lithium ion diffuse across this gap and offload their electrons at the other side, they stick to the electrode there, and can snap off as the whole thing expands and contracts. This removes some lithium ions from the system, thus reducing the total available charge in the battery.
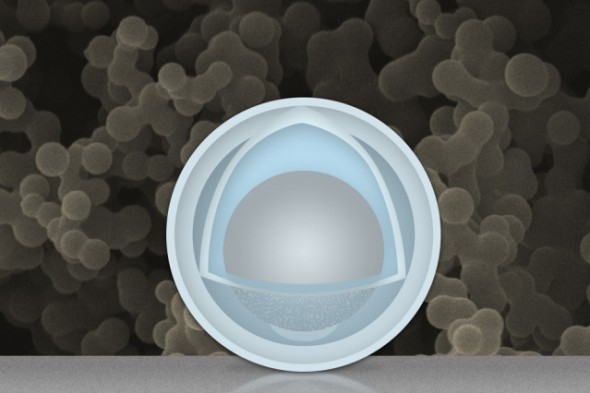
This expansion problem is one of the reasons graphite has been used for so long, since it undergoes relatively little change throughout the battery's use. In particular, aluminum has been a frequent candidate to replace graphite, but tends to get discarded because it expands and contracts too much, and because it builds up an unhelpful coating when exposed to air. Researchers from MIT were attempting to address this problem with different treatments for aluminum nanoparticles — and that work led them to bathe nanoparticles in a mixture of sulfuric acid and titanium oxysulfate, with the intention of replacing the aluminum oxide coating that results from reaction with the air with a more practical coating of titanium oxide.
The issue arose when the team accidentally left a sample of aluminum in the bath for several hours longer than their technique required. This resulted in an unforeseen egg-like nanoparticle design, in which a "yolk" of aluminum is covered in a "shell" of titanium dioxide. What's important is that there is some space between the yolk and the shell (where the metaphorical "white" would go), which allows the aluminum to expand and contract as it is wont to do without affecting the titanium shell around it. This means that the aluminum can react to the regular charge-discharge cycle without trapping and removing any of the lithium ions themselves.
The delay in removing the aluminum from the chemical bath did not result in the shell around the aluminum core, which would have been there anyway, but rather the shrinking of that core to a "yolk" with the all-important internal space. Though the team had not meant to create that unintentional chemical product, the researchers did have the insight needed to put the particles through their experimental paces, rather than simply throwing them out. Whether they did this because they thought it might produce something useful, or simply wanted to be diligent even with their failures, is unclear.
What is clear is that lithium ion batteries need a breakthrough like this to keep moving further into people's lives. Charge-discharge capacity has a lot to do with the lifetime costs of things like electric cars — if you could regularly drive an all-electric car for several years without much real risk of having to replace the battery pack, electric cars would become much more affordable over their full lifespans.